IMMUNOLOGY & MICROBIOLOGY DEPARTMENT
In this issue of the newsletter, we will be highlighting the research being carried out by
Immunology & Microbiology Department at DDI, led by Dr. Rasheed Ahmad.
RESEARCH UPDATE
Find out what is new within the Research Sector at DDI
Immunometabolism – A Promising Route For Drug Development
Published on 24/01/2020
Immunometabolism has emerged recently as a very exciting field of research in immunology that investigates the interplay between immunological and metabolic processes. Interest in this field is gaining thrust after recognition that incorrect metabolic remodeling underlies many abnormal immune responses associated with various metabolic diseases. The link between immune and metabolic responses is bidirectional, where inflammatory changes lead to chronic low-grade inflammation in obesity settings. Chronic tissue inflammation induces a wide range of effects on adipose tissue, muscle, liver, pancreatic islets, the gut, and the central nervous system (CNS). These inflammatory changes contribute to insulin resistance (adipocytes, muscle, liver), decreased insulin secretion (islets), dysbiosis and intestinal permeability (gut), and increased food intake (CNS). Integrated interaction/crosstalk between cells within a tissue and tissues within an organism that drives inflammation in the milieu of metabolic dysregulation. The most dominant forms of metabolic pathways are glycolysis, gluconeogenesis, glycogenolysis, lipogenesis, fatty acid oxidation and synthesis of amino acid (Figure 2).
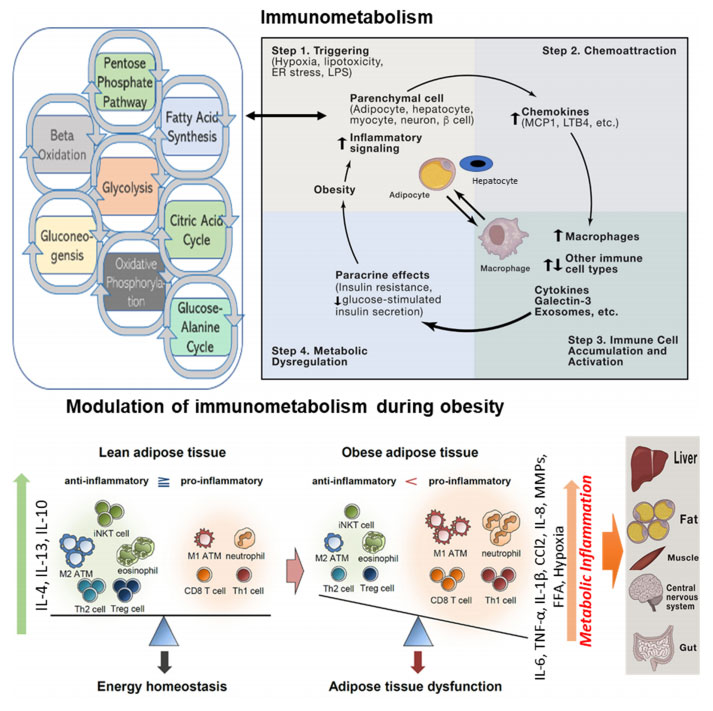

Figure 2: Obesity leads to various triggering events, such as ER stress, hypoxia, and lipotoxicity, which can initiate activation of proinflammatory pathways within tissue parenchymal cells (Step 1). As part of the activation mechanism in these parenchymal cells, they secrete a variety of chemokines (Step 2), which lead to chemotaxis and migration of macrophages, as well as other immune cell types, into the underlying tissue (Step 3). Overall, these immune cells take on a proinflammatory phenotype and secrete a number of factors (cytokines, galectin-3, miRNA-containing exosomes, etc.), which exert local paracrine effects to cause insulin resistance in adipocytes, hepatocytes, and myocytes, or decreased GSIS in b cells (Step 4). Bottom part of the Fig. shows the immune cells modulation in adipose tissue during obesity which leads to metabolic inflammation. Metabolic inflammation affects various tissues/organs. Adapted from Lee et al; Cell, 2018
Our research at DDI is broadly focused on different aspects of immunometabolism leading to metabolic inflammation. We have a longstanding interest in the immunological functions and signaling pathways of the immune cells and their interaction with diabetes related cells under the influence of overnutrition. We believe that understanding these pathways and modes of communication between the immune system and diabetes-related cells is important for translational studies. In addition, reprogramming immune responses through metabolic changes shows promise in the treatment of metabolic diseases.
One of our ongoing projects2 led by Dr Rasheed Ahmad focus on identifying the complex immunometabolic signaling networks and the epigenetic events that occur in the setting of overnutrition/obesity. In case of overnutrition/obesity, expanding adipose tissue releases inflammatory mediators called adipokines, and free fatty acids, into the circulation, which lead to low-grade chronic inflammation and insulin resistance. The co-expression of elevated FFAs levels and increased proinflammatory mediators such as TNFα, IL-6, IL-1β, IL-8, and MCP-1 exacerbates inflammatory responses by causing enhanced infiltration of immune cells into the peripheral metabolic tissues, such as fat, liver, and muscle. However, it remains to be seen how the interplay between various types of FFAs (saturated fatty acids/SFAs versus polyunsaturated fatty acids/PUFAs) and pro-inflammatory cytokines or chemokines induces, promotes and sustains metabolic inflammation. The emerging evidence from epigenetics has provided new insights into the pathogenesis of obesity/type 2 diabetes (T2D) and associated complications. We hypothesize that epigenetic changes driven by imbalances in high fat diet nutrients and their metabolites as well as their interaction with high levels of circulatory adipokines may play a key role in inducing, sustaining or promoting metabolic inflammation. We are using both in in vitro and in vivo approaches to test this hypothesis.
Our initial results show that different nutrients and cytokines (TNF-α, IL-6, IL-1β) change the epigenetic programming and promote/sustain inflammation, leading to insulin resistance.
Clinical Significance: Nutritional factors are of central importance to the development of obesity and induction of insulin resistance or development of T2D. High fat diets are a mixture of different lipids and molecules, and each of which may have distinct pathways. In obese/T2D individuals, high levels of saturated fatty acids (SFAs) and low levels of PUFAs are present in the circulation which has a direct impact on the disease progression. This study will investigate the obesity-related nutritional factors that could modulate gene expression by affecting epigenetic mechanisms and will also help to identify novel therapeutic strategies based on nutritional or pharmacological agents that can modify the epigenetic factors.



