DDI scientists presented three oral presentations and eight posters at the American Diabetes Association 84th Scientific Sessions in Orlando, FL, USA. Below are the abstracts from each department, detailing DDI’s most recent research findings.
Three abstracts and one oral presentation were submitted by the Microbiology & Immunology department:
Peroxisome proliferator-activated receptor delta (PPARδ): a key modulator in the pathogenesis of diabetes mellitus and mycobacterium tuberculosis co-morbidity
Halemah AlSaeed, Mohammed J. A. Haider, Fawaz Alzaid, Fahd Al-Mulla, Rasheed Ahmad, Fatema Al-Rashed
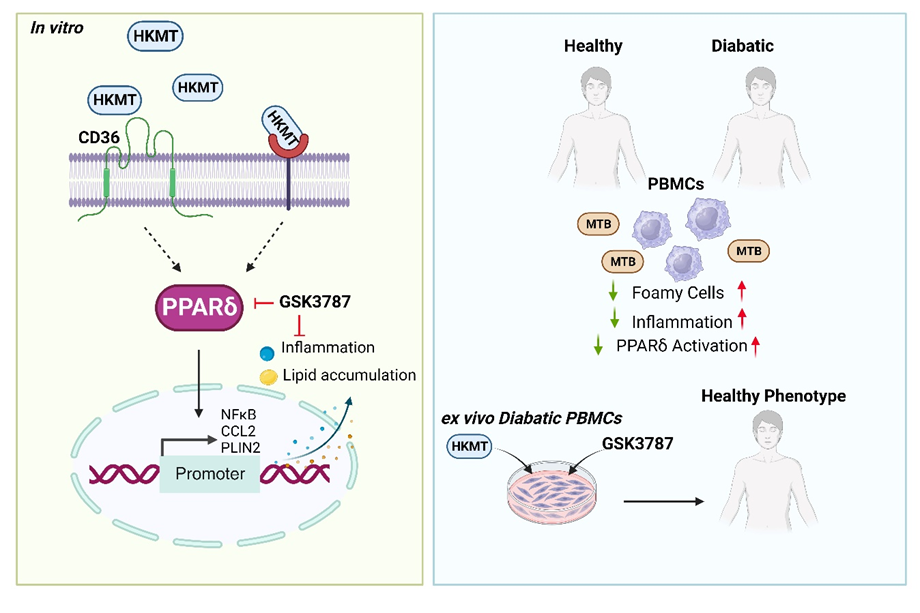
The interplay between lipid metabolism and immune response in macrophages is crucial in infectious diseases like tuberculosis (TB). This study explores how heat-killed Mycobacteria tuberculosis (HKMT) affects macrophage lipid metabolism and inflammation. HKMT activates the lipid scavenger receptor CD36, leading to lipid accumulation. However, inhibiting CD36 reduced lipid buildup but increased inflammation, shown by higher CCL2 secretion. This effect was linked to upregulation of PPARδ. Functional analyses revealed PPARδ’s key role in regulating lipid dynamics and inflammation, making it a potential therapeutic target. Additionally, peripheral blood mononuclear cells (PBMCs) from diabetic individuals, who are at higher risk for TB, showed increased PPARδ expression and inflammation. Targeting PPARδ in these cells reduced inflammation, suggesting a promising therapeutic strategy against TB, especially in diabetic patients.
Early-aged mice stay metabolically stable with High Fat Diet
Fatemah Bahman, Shihab Kochumon, Nadeem Akhter, Shaima Albeloushi, Reeby Thomas, Nermeen Abu Khalaf, Fatema Al-Rashed, Fahd Al-Mulla, Rasheed Ahmad
The relationship between obesity, aging, and metabolic disease risk is crucial. This research examines how aging affects the body’s response to high-fat diet (HFD) consumption and its impact on metabolic health. We hypothesize that aging influences adaptation to HFD, potentially affecting metabolic outcomes. We studied mice aged 4, 6, 8, and 10 weeks (6-8 mice per group) over 16 weeks on either a standard chow diet or HFD. Older mice gained weight more rapidly on HFD than younger mice, who seemed protected against HFD-induced obesity and had fewer inflammatory markers in adipose tissue. Characteristics of non-alcoholic fatty liver disease (NAFLD) were absent in younger mice on HFD. Liver staining with H&E and Oil Red O showed more lipid accumulation in older mice. Older mice also exhibited impaired glucose tolerance and reduced insulin sensitivity compared to younger mice on HFD. In conclusion, early aging significantly influences metabolic responses to HFD, suggesting potential strategies to prevent obesity and related complications. This research highlights the importance of age in dietary and metabolic health.
Sunflower Oil based HFD Feeding in Mice Leads to Gut Dysbiosis, Mild Steatohepatitis and Impaired Glycemic Control
Fatemah Bahman, Sardar Sindhu, Shihab Kochumon, Md. Zubbair Malik, Hossein Arefanian, Texy Jacob, Rasheeba Nizam, Reeby Thomas, Steve Shenouda, Fahd Al-Mulla, Rasheed Ahmad
High-fat diets (HFDs) affect gut microbiota and promote obesity, inflammation, and metabolic issues. Sucrose also contributes to gut dysbiosis. Fish and sunflower are considered healthy fat sources, but their effects on gut microbiota without sucrose remain unclear. We compared sucrose-free, sunflower high-fat diet (S-HFD) and fish high-fat diet (F-HFD) over 24 weeks in C57BL/6 mice, focusing on gut microbiota, obesity, hepatic inflammation, and insulin resistance. Both diets similarly affected body weight. 16S rRNA sequencing showed distinct gut microbial communities: Firmicutes were more abundant in S-HFD (40%) than F-HFD (3%), while Verrucomicrobia were higher in F-HFD (26%) compared to S-HFD (˂1%). Firmicutes/Bacteroidetes ratios were 1.32 for S-HFD and 2.45 for F-HFD. MicrobiomeAnalyst and MIAOME analyses identified that highly-abundant gut microbial taxa (g_Oscillospira, s_guilliermondii, g_Allobaculum, g_Yaniella, g_Ruminococcus, s_gnavus, g_Staphylococcus, g_Clostridium, g_Adlercreutzia, f_Aerococcaceae, g_Anaeroplasma, f_Mogibacteriaceae, f_Christensenellaceae, and o_RF39) in S-HFD mice had predicted associations with host genes related to metabolic disorders and pathways linked to inflammation and immune dysregulation. S-HFD mice showed increased body weight and fasting glucose, hepatic macrophage infiltration (F4/80 expression), macrovascular steatosis, lobular inflammation, and enhanced expression of genes associated with de novo lipogenesis (Acaca, Fasn, and Scd1) and monocyte chemotaxis (Ccl2), compared with F-HFD mice. In conclusion, S-HFD feeding induces gut dysbiosis which is consistent with moderate steatohepatitis and impaired glycemic control.
Sustained TNF-α transcription triggered in obesity through H3K9/K18 acetylation is associated with metabolic impairment
Rasheed Ahmad, Fatemah Bahman, Shihab Kochumon, Texy Jacob, Fatema Al-Rashed, Ajit Wilson, Hossein Arefanian, Reeby Thomas, Nadeem Akhter, Nourah Al-Mansour, Shaima Albeloushi, Ashraf Al Madhoun, Sardar Sindhu, Fawaz Alzaid, Fahd Al-Mulla
Obesity-induced metabolic inflammation, often triggered by high-fat diets (HFD) rich in saturated fats, leads to various metabolic disorders. While TNF-α’s role in metabolic dysfunction is clear, the pathways regulating its transcription in obesity are not fully understood. To investigate this, we used a murine model of diet-induced obesity (DIO). Male C57BL6/J mice were fed either a palmitate oil-based HFD or a control diet (CD) for 16 weeks. Compared to the CD group, DIO mice showed elevated TNF-α levels and increased acetylation of histone H3 lysine 9 and 18 (H3K9/K18) in adipose tissue and liver. Chromatin Immunoprecipitation (ChIP) assays revealed higher levels of permissive acetylation marks H3K9/K18 in the TNF-α promoter in DIO mice. This increased TNF-α expression correlates with histone acetylation changes, body weight gain, insulin resistance, and liver steatosis. Our findings suggest that DIO sustains TNF-α gene transcription via chromatin remodeling, contributing to metabolic dysfunction. Acetylation of H3K9/H3K18 may serve as prognostic factors for metabolic impairment and liver steatosis in obesity.